Translate this page into:
Synchronous Spontaneous Cochlear Emissions in Neonates and Adults: A Comparative Study
Address for correspondence Jim Saroj Winston, M.Sc., Department of Audiology and Speech Language Pathology, Nitte Institute of Speech and Hearing, Nitte (Deemed to be) University, Deralakatte, Mangalore 575018, Karnataka, India (e-mail: jim.saroj@nitte.edu.in).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
The primary objective of the current study was to characterize synchronous spontaneous otoacoustic emissions (SSOAEs) in adults and neonates. It was also interesting to compare the prevalence, frequency, and amplitudes of SSOAE in neonates and adults.
Materials and Method
A prospective comparative study design was employed in which synchronized SSOAEs were recorded binaurally from 92 neonates and 100 adults using an Echoport ILO 292 OAE analyzer. The recorded spectrum was analyzed for the number, amplitude, spectral distribution, and prevalence of SSOAEs.
Statistical Analysis
The data were subjected to descriptive and inferential statistics using JASP version 0.16.1.0. A chi-squared test was used to compare the prevalence of SSOAEs in the test population. The Shapiro-Wilk test for normality was administered to check the data distribution. The nonparametric Mann-Whitney U test and parametric independent t-test were used to compare the amplitude and frequency data.
Results
The findings revealed a higher prevalence of SSOAEs in neonates (42.8%) compared with adults (18%). The analysis also showed that the multiple-frequency SSOAEs were more prevalent than single-frequency SSOAEs in adults and neonates. The percentage of SSOAE occurrence was highest in the 2- to 3-kHz bin for adults, whereas in neonates, most SSOAEs occurred between 3 and 4 kHz. The results showed that the SSOAE amplitude across frequency bands was significantly higher in newborns compared with adults in all the frequency bins.
Conclusion
The present study revealed a lesser prevalence of SSOAE in adults and neonates than in earlier reports. However, no difference in the spectral characteristics was observed.
Keywords
SSOAE prevalence
neonates
Spectral distribution
SSOAE number
SSOAE amplitude
Introduction
Otoacoustic emissions, generated without external stimuli, are termed spontaneous otoacoustic emissions (SOAE) and indicate normal outer hair cell functioning.1,2 Although the exact mechanism of its generation is unknown, it likely originates from nonlinear outer hair cell activity at the place in the cochlea tuned to its frequency3 and due to minor structural irregularities of the cochlea, which are not significant enough to affect audiometric thresholds.4 Traditionally, SOAEs have been elicited using two methods, in the absence of external stimuli (SOAEs) and synchronized with the silence subsequent to stimulus delivery for transient evoked otoacoustic measurement, commonly known as synchronous spontaneous otoacoustic emissions (SSOAEs).5
Despite its early discovery and widespread interest of researchers, the clinical utility of SOAE remains limited due to its lower prevalence, lack of consensus in characterization, and lack of research exploring factors affecting SOAEs. A plethora of procedural and subject-related factors are known to influence SSOAEs. Interestingly, the prevalence data have risen over the years with advancements in the sensitivity of microphones and procedures. The prevalence of SOAEs is known to be directly linked with age, with a higher prevalence (64%) in neonates compared with adults (30%).6 SOAE amplitudes and their frequency distribution are also known to be age-dependent. Burns et al7 and Braun8 reported higher SOAE amplitudes and a frequency distribution concentrated upward of 2,500 Hz in neonates compared with adults. Further, few studies have reported the influence of race on SOAEs.6,9,10 Their reports reveal a significant effect of race on the prevalence, amplitude, and spectral distribution of SOAEs. In the Indian context, the prevalence of SOAEs in adults is 43.5%,11 which is less than the higher prevalence of 61% reported in American adults.7 The prevalence reports vary among infants from 38%12 to 62%.7 The reported prevalence of SOAEs is higher among neonates, ranging from 78%13 to 86.5%.14
The potential clinical applications of SOAEs are well documented in the literature. In a recent study, Mertes15 demonstrated the potential utility of synchronized SSOAEs over transient-evoked OAEs (TEOAEs) in detecting the medial olivocochlear bundle functioning. SOAE suppression allows the objective evaluation of cochlear frequency selectivity by determining the suppression tuning curves.16 A case study by Penner17 also illustrates the evidence for the possible link between SOAEs and tinnitus generation.
While some work has explored the prevalence of SOAEs, the lack of consensus in the earlier prevalence reports and underexplored age-related factors highlight the need to investigate SSOAE in adults and neonates. Additionally, the characterization of SOAEs is relevant in addressing gaps in existing knowledge. Further, many studies in the literature that have reported the prevalence and characteristics of SOAE have used the traditional method of recording SOAE in a nonsynchronized manner. Synchronized SOAEs are explored less regarding their prevalence and characteristics, especially in neonates. Given that most commercially available OAE devices now incorporate SSOAEs as part of their recording protocols and the lack of studies focusing on neonates using SSOAEs, the current study was designed to explore the characteristics of SSOAEs in both adults and neonates. It was also interesting to compare the prevalence and amplitude of SSOAE in Indian neonates and adults.
Methods
The present study employed a between-group comparative research design and was conducted in the audiology and speech-language pathology department of a tertiary hospital in Karnataka, India. The recording environment had ambient noise levels well within the limits of ANSI 3.0.18
Participants
Ninety-two neonates (<3 days old; mean age = 2.62 ± 0.34 days; 42 females; 184 ears) and 100 adults (age 18–25 years; mean age = 22.67 ± 2.12 years; 62 females; 200 ears) were recruited as participants based on convenience sampling. All neonates in the present study had Appearance, Pulse, Grimace, Activity, and Respiration (APGAR) scores greater than 4 and 6, respectively, at 1 and 5 minutes after birth.19 Further, all the neonates were screened using the high-risk register (HRR) for medical persons tapping prenatal, perinatal, and postnatal history.20 A detailed audiological history was obtained from the adult participants to rule out the presence of any significant medical or otological history. All the participants selected for the study had normal outer hair cell functioning, confirmed using 83 dB peak SPL click-evoked TEOAEs at test frequencies of 1,000, 1,414, 2,000, 2,828, and 4,000 Hz. The TEOAEs were considered present if the signal-to-noise ratio (SNR) at three adjacent test frequencies was greater than 6dB.11 Before testing, the ear canals were visually inspected using an otoscope (Welch Allyn LED Fiber-Optic) to ensure there was no debris or obstruction.
Procedure
The TEOAEs and SSOAEs were recorded using an Echoport ILO 292 (Otodynamics Inc, Switzerland) in a sound-treated room. The adult participants were made to sit comfortably on a chair, whereas the neonates were made to lie on a cradle while recording. The OAE recording probe was inserted deep into the participant's ear canal, and a hermetic seal was ensured. To record TEOAEs, the stimulus sound pressure level in the ear canal was adjusted to 80 dB peak SPL using the auto-adjust function, and 260 sweeps of clicks were presented. After the stimulus was presented, the sound level in the ear canal was recorded for 20 milliseconds. Once TEOAEs were confirmed, low-level clicks were given to record synchronized SOAEs and a recording window of 80 milliseconds was used. The recorded power spectrum in the 60- to 80-millisecond window was analyzed for the parameters of interest of SSOAE as TEOAEs elicited by a click do not persist after 20 milliseconds. This recording method is a default setting on the ILO system by Otodynamics for recording SOAE.
Analysis
Fig. 1 shows a few SSOAE power spectrum samples recorded from the study population. The SSOAEs were considered present if the absolute amplitude was above the noise floor by 3 dB SPL at each frequency of occurrence.11 The number of individual SSOAE peaks in a recording was counted and used to classify the ear as having multiple and single SSOAEs. The power spectrum of SSOAE was categorized into five frequency bins with a bandwidth of 1,000 Hz from 1 up to 6 kHz. The frequency of occurrence of SSOAE in each frequency bin (1–2, 2–3, 3–4, 4–5, and 5–6 kHz) was used to obtain the spectral distribution of SSOAEs in adults and neonates separately. Further, the amplitude of the SSOAE was measured manually for each peak from the recorded power spectrum using a curser kept on the tip of the SSOAE.
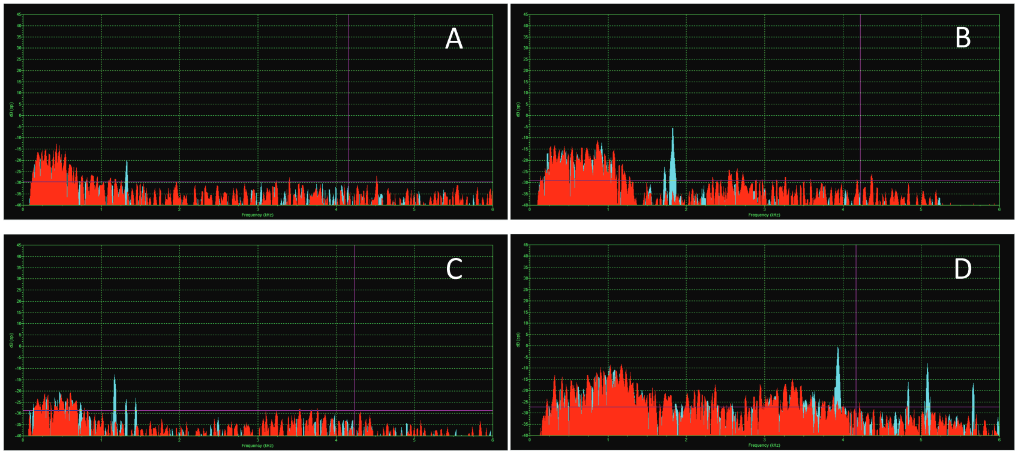
- Sample power spectrum recorded in the present study showing (A) single, (B) two and (C) three synchronous spontaneous otoacoustic emission (SSOAE) peaks in adults, and (D) recording from a neonate with four SSOAE peaks.
Statistical Analysis
All the above parameters are tabulated and subjected to further descriptive and inferential statistical analysis using JASP software version 0.16.1.0.21 Descriptive statistics were performed to obtain the prevalence of SSOAE, unilateral and bilateral SSOAEs, multiple and single OAEs, and mean amplitude of SSOAEs. The Shapiro-Wilk test for normality was administered to check the data distribution, which revealed normal distribution (p >0.05) for absolute amplitude and frequencies of occurrence. A paired t-test was administered using SSOAE amplitude and frequency data to check the significance of the ear effect. A chi-squared test was performed to compare the prevalence of SSOAE in adults and neonates. The prevalence of unilateral versus bilateral SSOAEs and single versus multiple SSOAEs was compared between adults and neonates using the chi-squared test. The Mann-Whitney U test and independent t-test were used to compare the amplitude between adults and neonates across five frequency bins.
Results
A total of 192 participants (100 adults and 92 neonates) recruited in the study resulted in the data/recordings from 384 ears. The data from 15 neonates were discarded due to higher noise levels at the probe microphone due to biological factors. As the data were normally distributed, as revealed by the Shapiro-Wilk test (p < 0.05), parametric tests were considered for the inferential analysis. Initially, the paired sample t-test was used to test for significant ear effect. The results revealed no significant ear effect on the amplitudes of SSOAEs in adults (t(36) = 1.51; p = 0.14) or neonates (t(71) = 0.12; p = 0.90). Also, no statistically significant ear effect was observed on the SSOAE frequencies in adults (t(36) = −0.75; p = 0.45) or neonates (t(71) = −1.53; p = 0.12). Since the paired sample t-test revealed no significant ear effect for neonates and adults, the SSOAE amplitude and frequency data from the right and left ears were combined and considered for further analysis.
Prevalence of SSOAEs
The SSOAEs were present in 18% of adults (36 of 200 ears tested) and 42.8% of neonates (66 of 154 ears tested). The chi-squared test revealed that the prevalence of SSOAE was significantly higher in neonates compared with adults (X2 = 20.201; p = 0.001). Fig. 2A depicts the prevalence of single and multiple SSOAEs in the adult and newborn groups. Multiple SSOAEs were significantly more prevalent than single SSOAEs in the adult (x2 = 19.45; p = 0.02) and newborn (X2 = 18.74; p = 0.01) groups.
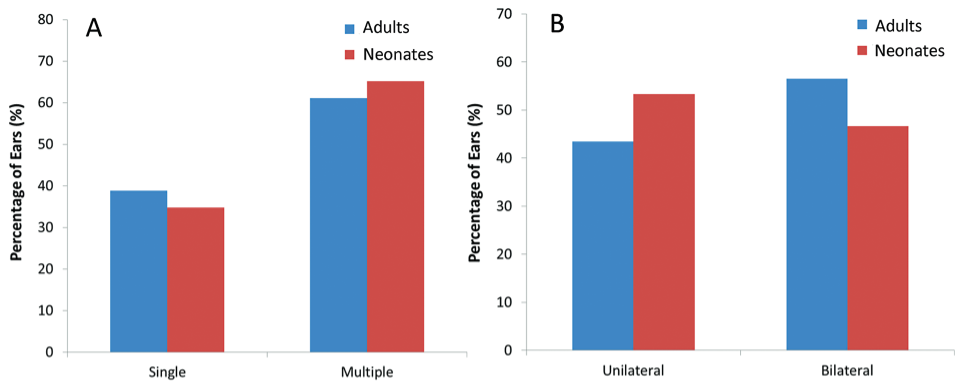
- Prevalence of (A) single versus multiple synchronous spontaneous otoacoustic emission (SSOAE) and (B) unilateral versus bilateral SSOAEs in adults (n = 33 ears) and neonates (n = 174 ears).
Between-group comparisons showed no significant difference in the prevalence of single (X2 = 0.591; p = 0.85) and multiple (X2 = 0.532; p = 0.83) SSOAEs. However, Fig. 2A reveals that multiple SSOAEs were more prevalent in the newborn group, and single SSOAEs were more prevalent in the adult group. The chi-squared test for the 2*2 contingency table showed no significant difference in the prevalence of unilateral versus bilateral SOAEs between adults and neonates (X2 = 0.591; p = 0.44; Fig. 2B).
Spectral Distribution
The spectral distribution of SSOAE in adults and neonates is depicted in Fig. 3A. Results showed that the percentage of SSOAE occurrence was highest for adults in the 2- to 3-kHz bin. In contrast, most of the SSOAEs were located in the 3- to 4-kHz bin in neonates. Z scores for proportions were used to compare the group differences in the prevalence of SSOAEs in the frequency bins, and the results are tabulated in Table 1.
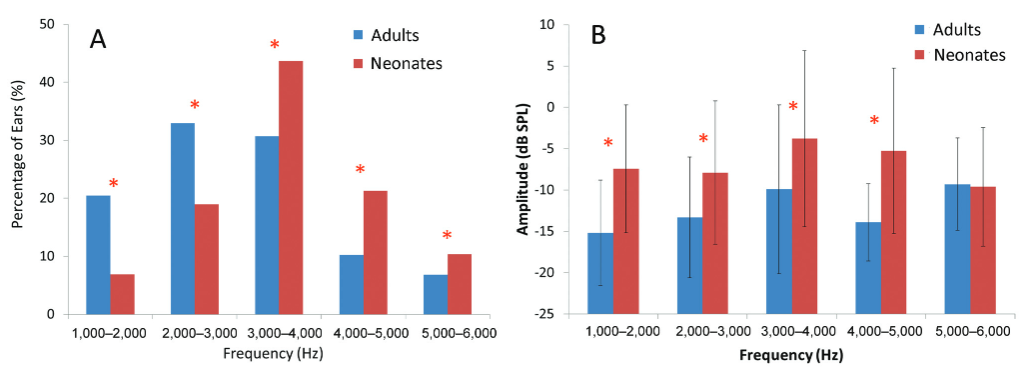
- Spectral distribution of (A) synchronous spontaneous otoacoustic emissions (SSOAEs) and (B) the amplitude of SSOAEs in adults (n = 33 ears) and neonates (n = 174 ears). The asterisk (*) symbol indicates significance at p < 0.05.
| Spectral distribution | Spectral amplitudes | |||
|---|---|---|---|---|
| Frequency bins | Test statistic | p | Test statistic | p |
| 1,000–2,000 Hz | Z = 9.08 | <0.001 | t = −3.005 | <0.001 |
| 2,000–3,000 Hz | Z = 10.81 | <0.001 | U = 309.00 | <0.001 |
| 3,000–4,000 Hz | Z = 7.52 | <0.001 | U = 646.00 | <0.001 |
| 4,000–5,000 Hz | Z = 2.691 | 0.007 | t = −0.09 | 0.012 |
| 5,000–6,000 Hz | Z = 2.96 | 0.002 | t = −0.239 | 0.812 |
Absolute Amplitude
As the amplitude data of 2 to 3 and 3 to 4 kHz were not normally distributed, the Mann-Whitney U test was used to compare the amplitude between adults and neonates in these frequency bins. In contrast, the independent t-test was performed for other bins. The results showed that the SOAE amplitude across frequency bands was significantly higher in neonates than in adults in all the frequency bins except 5 to 6 kHz, as depicted in Fig. 3B. Table 1 summarizes the test statistics and the level of significance data of the Mann-Whitney U test and t-test comparing the SSOAE amplitudes across frequency bands for adults and neonates.
Discussion
The findings of the present study reveal a significantly higher prevalence of SSOAE in neonates than in adults. The higher prevalence of SSOAEs in neonates is in line with the previous reports12 and can be attributed to the maturational influence of the efferent system on the outer hair cell activity.22 However, the prevalence of SSOAE in neonates in the current study is relatively lesser than that reported in previous studies.7,13 This variance could be due to the timing of SSOAE recording: the current study recorded SSOAEs within 3 days of birth, whereas Kok et al13 recorded SSOAEs within 10 days of birth and Burns et al7 recorded SSOAEs between 10 and 31 days after birth. This suggests a link between SOAEs and maturational changes, as SOAEs are considered a unique phenomenon resulting from spontaneous outer hair cell hyperactivity during the infantile period.23
Further evidence in the literature indicates significant changes in otoacoustic emission latency with postconception age, suggesting that SOAEs may serve as a potential marker of maturational changes in cochlear tuning.24 Furthermore, analysis of the number of SSOAEs revealed that multiple SSOAEs were more likely to be present in the newborn group. In contrast, the adult group exhibited a higher prevalence of single SSOAEs. The higher prevalence of multiple SSOAEs in neonates aligns with earlier reports and could be attributed to the maturational aspects that occur at the beginning of life.13,22
An analysis of the spectral distribution of SSOAEs in neonates and adults showed a significantly higher prevalence of SSOAEs in a relatively higher frequency region in neonates (3–4 kHz) than in adults (2–3 kHz). The greater concentration of SSOAEs in the relatively higher frequency region in neonates is consistent with the previous reports.7,12,13,25 It could be attributed to the shorter ear canal length in neonates leading to the shift in resonance to a relatively higher frequency. In addition to the contribution of the middle ear and external ear canal resonance, Morlet et al suggested that the higher concentration of SOAEs in higher frequencies in neonates than adults could be because of additional noise below 2,500 Hz when testing neonates or maturational changes within the cochlea or efferent system.22
The present study also reports a significantly higher amplitude of SSOAEs in neonates than in adults, which agrees with existing literature.13,14,22,25 The higher amplitudes in neonates may be due to the smaller ear canal volume, resulting in higher sound pressure levels at the ear canal. It could also be attributed to the incomplete maturation of auditory efferent pathways, which may lead to the disinhibition of the cochlear amplifier effect, resulting in higher amplitudes. Future studies on SSOAEs could consider gender as a variable that could plausibly have influenced the present findings.
Conclusion
The present study explored the SSOAE characteristics in neonates and adults. The results reveal a higher prevalence of SSOAE in neonates than in adults. Multiple SOAEs were more prevalent than single-frequency SSOAEs in both groups. The prevalence of SSOAE was highest in frequency bins of 3 to 4 and 2 to 4 kHz in neonates and adults, respectively. Further, the amplitude of SSOAE was significantly higher in neonates than in adults except at 5 to 6 kHz. The present study revealed a lesser prevalence of SSOAE in adults and neonates than in earlier reports. However, no difference in the spectral characteristics was observed.
Acknowledgments
We thank the management of Nitte Deemed to be University, Mangalore, and all the study participants for facilitating this study at all stages.
Ethical Declaration
The authors hereby declare that the research article titled “Synchronous Spontaneous Cochlear Emissions in Neonates and Adults: A Comparative Study” has been conducted following clauses of the Declaration of Helsinki (2013).
Informed Consent
Prior informed consent was obtained from the adult participants and the legal caretakers of the newborns regarding their willingness to participate in the study through a Google form circulated via e-mail.
Authors' Contributions
P.K.E., J.S.W., S.G., D.R. contributed to the design and concept of the study. D.R., S.H., and I.P. contributed to acquisition of data. P.K.E., J.S.W., and S.G. drafted the manuscript and interpreted the results.
Conflict of Interest
None declared.
References
- Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea. Arch Otorhinolaryngol. 1979;224(1-2):37-45.
- [Google Scholar]
- An integrated view of cochlear mechanical nonlinearities observable from the ear canal In: Mechanics of Hearing. Dordrecht: Springer; 1983. p. :75-82. In:
- [Google Scholar]
- Spontaneous cellular vibrations in the guinea-pig temporal-bone preparation. Br J Audiol. 1993;27(02):79-83.
- [Google Scholar]
- Otoacoustic emissions, travelling waves and cochlear mechanisms. Hear Res. 1986;22(1-3):95-104.
- [Google Scholar]
- Can synchronized otoacoustic emissions really be attributed to SOAEs? Hear Res. 1994;80(02):141-145.
- [Google Scholar]
- Characterization of spontaneous otoacoustic emissions in full-term newborns. Int J Pediatr Otorhinolaryngol. 2014;78(12):2286-2291.
- [Google Scholar]
- Prevalence of spontaneous otoacoustic emissions in neonates. J Acoust Soc Am. 1992;91(03):1571-1575.
- [Google Scholar]
- A retrospective study of the spectral probability of spontaneous otoacoustic emissions: rise of octave shifted second mode after infancy. Hear Res. 2006;215(1-2):39-46.
- [Google Scholar]
- Spontaneous otoacoustic emissions in different racial groups. Scand Audiol. 1993;22(01):3-10.
- [Google Scholar]
- Spontaneous otoacoustic emissions in individuals with auditory neuropathy spectrum disorder. Audiol Med. 2012;10(01):50-54.
- [Google Scholar]
- Incidence of spontaneous otoacoustic emissions in children and infants. J Acoust Soc Am. 1985;78(03):931-935.
- [Google Scholar]
- Aspects of spontaneous otoacoustic emissions in healthy newborns. Hear Res. 1993;69(1-2):115-123.
- [Google Scholar]
- Properties of SOAEs and their correlation with TEOAEs in neonates. Korean J Otorhinolaryngol-Head Neck Surg. 1999;42:1359-1363.
- [Google Scholar]
- Medial olivocochlear reflex effects on synchronized spontaneous otoacoustic emissions. J Acoust Soc Am. 2020;147(03):EL235-EL240.
- [Google Scholar]
- Frequency selectivity of tonal language native speakers probed by suppression tuning curves of spontaneous otoacoustic emissions. Hear Res. 2020;398(108100):108100.
- [Google Scholar]
- Audible and annoying spontaneous otoacoustic emissions. A case study. Arch Otolaryngol Head Neck Surg. 1988;114(02):150-153.
- [Google Scholar]
- ANSI/ASA S3.1-1999 (R2018): Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. New York, NY: Acoustical Society of America
- The Apgar score: evolution, limitations, and scoring guidelines. Birth. 1991;18(02):83-92.
- [Google Scholar]
- High Risk Register (HRR): For Medical Persons Screening for Communication Disorders in Children. Mysore: All India Institute of Speech and Hearing; 2012.
- JASP: graphical statistical software for common statistical designs. J Stat Softw. 2019;88(02):1-7.
- [Google Scholar]
- Spontaneous otoacoustic emissions in preterm neonates: prevalence and gender effects. Hear Res. 1995;90(1-2):44-54.
- [Google Scholar]
- Spontaneous otoa-coustic emissions in two infants. Acta Otolaryngol. 2009;129(04):399-404.
- [Google Scholar]
- Transient evoked otoacoustic emission latency and estimates of cochlear tuning in preterm neonates. J Acoust Soc Am. 2008;124(05):2984-2994.
- [Google Scholar]
- The properties of spontaneous and evoked acoustic emissions in neonates and children: a preliminary report. Arch Otorhinolaryngol. 1989;246(05):249-251.
- [Google Scholar]







