Translate this page into:
Noninvasive Encapsulated Papillary Carcinoma of the Breast with Nodal Micrometastasis: A Rare Entity
Address for correspondence Shubha Padmanabha Bhat, MD (Pathology), Additional Professor, Department of Pathology, KS Hegde Medical Academy, Nitte (Deemed to be University), Mangalore, Karnataka, India (e-mail: bhatshubha_257@nitte.edu.in).
This article was originally published by Thieme Medical and Scientific Publishers Pvt. Ltd. and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Papillary neoplasms of the breast are rare and often pose diagnostic challenges to pathologists in routine practice. Encapsulated papillary carcinoma (EPC) of the breast is a rare type of papillary neoplasm. This tumor presents in postmenopausal women. Sonomammogram will aid in locating and identifying it as a cystic lesion. Fine needle aspiration cytology will help in diagnosing the papillary nature of the lesion. Surgical excision is the treatment of choice. Histopathology is necessary for accurate diagnosis. In rare circumstances, noninvasive EPC can have axillary nodal metastasis. We report the case of a 52-year-old woman who came with complaints of a lump in the left breast. This lump was radiologically suspected to be a phyllodes tumor. Fine needle aspiration cytology helped in identifying the lesion as papillary neoplasm. Histopathological examination revealed noninvasive encapsulated papillary carcinoma with axillary lymph node micrometastasis.
Keywords
encapsulated papillary carcinoma
histopathology
micrometastasis
Introduction
Encapsulated papillary carcinoma (EPC) is a rare breast cancer accounting for approximately 1 to 2% of all breast carcinomas in women. EPC presents in postmenopausal women mainly in the seventh decade of life. EPC has also been described in males. Patients present with a lump in the breast under the nipple region with or without nipple discharge.1 On sonomammogram, it appears as a well-defined, round to oval heterogenous mass with varying signal intensity depending on the cyst fluid content. Fine needle aspiration cytology (FNAC) can aid in the diagnosis by suspecting a papillary lesion2. Complete surgical excision of the lesion with extensive sampling of the surrounding breast is essential for treatment and for assessment of local recurrence.3 Grossly, EPC is observed as a friable mass within cystic spaces. On histopathology, EPC shows a cystically dilated duct surrounded by a fibrous capsule with intraluminal arborization of the fibrovascular stroma that is covered entirely by atypical epithelium with low or intermediate nuclear grade and devoid of myoepithelial cells in the papillae and at the periphery. Frank invasion is defined as the presence of neoplastic elements that permeate beyond the fibrous capsule with an irregular infiltrative appearance.2 EPC even without invasion can lead to axillary metastasis in rare circumstances.4 Incidence of EPC with lymph node involvement is 7%. Immunohistochemically, EPC is strongly positive for estrogen receptors (ER) and progesterone receptors (PR) and negative for human epidermal growth factor receptor 2 (HER2). In the absence of associated areas of infiltrating carcinoma, EPC has a very favorable prognosis. Herein, we report the case of a 52-year-old female patient who presented with a lump over the left breast. Computed tomography (CT) scan suspected the mass as phyllodes tumor and FNAC helped in diagnosing it as a papillary neoplasm. A simple mastectomy with axillary dissection was performed, histopathology of which showed EPC without invasion with two axillary lymph nodes showing micrometastasis.5
Case History
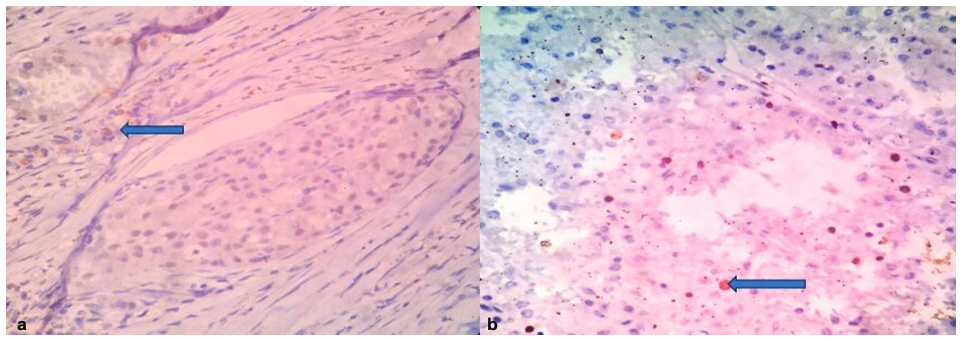
A 52-year-old woman presented with a history of a lump over the left breast for 6 months. Initially, it was the size of a lemon, which slowly increased to the present size. It was associated with mild pain over the left breast. There was no history of any discharge from the nipple. A chest X-ray revealed a well-defined soft-tissue density in the left chest wall (Fig. 1a). Sonomammogram showed a multilobulated lesion measuring 12.3 cm × 11.2 cm × 9.4 cm with solid and cystic areas in the left breast involving all the quadrants. A probable diagnosis of phyllodes was suggested. A CT scan of the thorax was done, which showed solid to cystic mass with enlarged axillary lymph nodes in the left breast and was suspected to be phyllodes tumor (Fig. 1b). An FNAC of the left breast showed highly cellular smears with ductal epithelial cells arranged in the papillae with fibrovascular cores. They had round to oval uniform nuclei, inconspicuous nucleoli, abundant vacuolated cytoplasm, eccentric nuclei with focal mild atypia (Fig. 2). According to the International Academy of Cytology (IAC) Yokohama system, a category 4, suspicious finding with probable diagnosis of papillary neoplasm was made. Other laboratory investigations such as complete blood count, liver function test, and coagulation profile were within normal limits. A left simple mastectomy with axillary dissection was performed, and the specimen was sent for histopathological examination. Grossly, we received a left mastectomy specimen measuring 20 cm × 16 cm × 7.5 cm with skin measuring 17 cm × 13.3 cm. On the outer surface, a soft cystic lump was palpable over the lower outer quadrant and upper outer quadrant approximately measuring 4 cm × 3.2 cm at the 2 to 4 o'clock position. Cut surface revealed a well-encapsulated, multilobulated, cystic tumor measuring 11 × 8.2 × 5.1 cm filled with necrotic and hemorrhagic areas ( Fig. 3). A total of 13 lymph nodes were isolated. Histopathology of the tumor showed tumor cells arranged in a papillary pattern, as well as tubules, a cribriform pattern, and in singles. The papillae were slender and branching having a delicate fibrovascular stalk. They were lined by a pseudostratified layer of relatively mono-morphic cells having round to oval nucleus of low to intermediate grade. Chromatin was fine and cytoplasm was moderate. There were no myoepithelial cells lining the papillae. Interpapillary stroma was filled with tumor cells in the cribriform pattern containing intraluminal secretion. Tumor cells were also seen in singles having eccentric nucleus and vacuolated cytoplasm giving a signet ring-like appearance. At the periphery, there were papillae displaying high-grade pleomorphism having round to oval nuclei with dispersed chromatin and prominent nucleoli. Four to five mitoses/10 hpf were noted. Intervening stroma showed fibrosis, hemosiderin, and lymphoplasmacytic infiltrate. Large areas of hemorrhagic necrosis were seen secondary to infarction. The tumor cells were well confined within a fibrous capsule, which were lined by epithelial cells (Fig. 4). Two out of 13 lymph nodes identified showed micrometastasis (Fig. 5). Histopathological features were suggestive of noninvasive EPC, high grade with two lymph nodes showing micrometastasis. Pathological stage was pTis, pN1mi. On immunohistochemistry, ER was positive, PR and HER2 were negative, andKi-67 showed 22% proliferation rate (Fig. 6). Allred scoring was used to score ER as 2 +. Myoepithelial marker was not performed to rule out invasion as histologically no invasion was noted. The patient has finished four cycles of chemotherapy and is doing well.
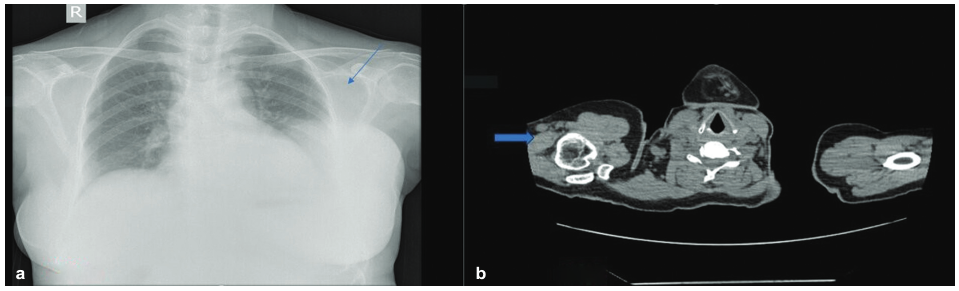
- (a) Chest X-ray showing a radiodense lesion in the left breast (blue arrow). (b) Computed tomography (CT) of the thorax showing an isoechoic lesion in the left breast (blue arrow).
![(a, b) Fine needle aspiration cytology (FNAC) showing ductal epithelial cells arranged in the papillae with fibrovascular cores, cells showing round to oval uniform nuclei, inconspicuous nucleoli, abundant vacuolated cytoplasm, eccentric nuclei with focal mild atypia (blue arrow; Papanicolaou [PAP] stain 10x and May Grünwald-Giemsa [MGG] stain 10x, respectively).](/content/192/2025/15/Supplement 1/img/JHASNU-15-S1-S101-g2.png)
- (a, b) Fine needle aspiration cytology (FNAC) showing ductal epithelial cells arranged in the papillae with fibrovascular cores, cells showing round to oval uniform nuclei, inconspicuous nucleoli, abundant vacuolated cytoplasm, eccentric nuclei with focal mild atypia (blue arrow; Papanicolaou [PAP] stain 10x and May Grünwald-Giemsa [MGG] stain 10x, respectively).
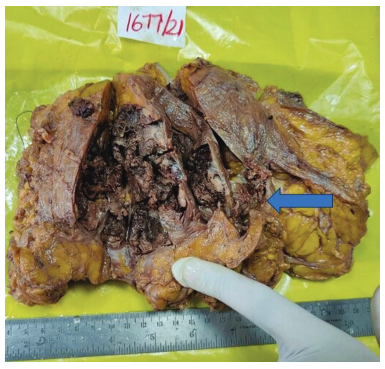
- Gross showing cut surface of the lesion showing a well-encapsulated, multilobulated, cystic friable mass filled with necrotic and hemorrhagic areas (blue arrow).
![(a) A well-encapsulated tumor showing tumor cells arranged in papillary, cribriform, and tubular pattern showing the capsule (blue arrow; hematoxylin and eosin [H&E] stain, 10x). (a) No myoepithelial cells at the tumor margin (blue arrow; H&E stain, 40x).](/content/192/2025/15/Supplement 1/img/JHASNU-15-S1-S101-g4.png)
- (a) A well-encapsulated tumor showing tumor cells arranged in papillary, cribriform, and tubular pattern showing the capsule (blue arrow; hematoxylin and eosin [H&E] stain, 10x). (a) No myoepithelial cells at the tumor margin (blue arrow; H&E stain, 40x).
![Lymph node showing papillary micrometastasis (blue arrow; hematoxylin and eosin [H&E] stain, 10x).](/content/192/2025/15/Supplement 1/img/JHASNU-15-S1-S101-g5.png)
- Lymph node showing papillary micrometastasis (blue arrow; hematoxylin and eosin [H&E] stain, 10x).
- (a) Estrogen receptor (ER) 2+ positivity (blue arrow; 40x). (b) Ki-67: 22% positivity (blue arrow; 40x).
Discussion
EPC presents in postmenopausal women, mainly during the seventh decade of life. It is a rare breast cancer that accounts for only 1 to 2% of all breast cancers, with incidence of nodal metastasis being even rarer at only 7% of all cases. EPCs show16q loss, 16p gains, and 1q gains. The prevalence of phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutation is like that in ER-matched and grade-matched Invasive breast carcinoma (IBCs) (NST - No special type). PAM50 subtyping classifies most cases as luminal A and a minority as luminal B.6 The most common sites are central and subareolar region. The patient presents with a lump in the breast, with or without nipple discharge.1 Imaging features are often nonspecific and there are no specific imaging features that differentiate EPCs from other papillary lesions. The classic appearance is a complex solid and cystic mass on sonomammogram with a Breast Imaging-Reporting and Data System (BIRADS) 4 suspicious designation.3 In the present case, a 52-year-old woman presented with a lump in the left breast and sonomammogram suggested a solid cystic lesion, with a suspicion of phyllodes. A thorax CT showed an isoechoic lesion in the left breast with a probable diagnosis of phyllodes tumor. FNAC shows cellular smears, with tumor cells arranged in papillae with slender fibrovascular stalks with a ramifying appearance, the presence of cell balls, increased numbers of single cells with mild atypia, cytoplasmic preservation, and columnar morphology. In our case, FNAC helped in diagnosing it as a papillary lesion.
Complete surgical excision of the lesion, with extensive sampling of the surrounding breast tissue, is essential for the treatment and assessment of local recurrence risk. Grossly, EPC is observed as a friable mass within a cystic space. A simple mastectomy with axillary dissection was performed in the present case. Grossly, a well-encapsulated, multilobulated, cystic tumor was noted. Histopathologically, the tumor consists of a papillary mass within a cystic space; less often, it is composed of an aggregate of close nodules. The tumor has a rounded, pushing border and is typically surrounded by a fibrous capsule of varying thickness. The lesion consists of multiple delicate fibrovascular stalks covered by a mono-morphic population of neoplastic epithelial cells with low- or intermediate-grade nuclei. The epithelial cells are arranged in single or multiple cell layers, and they can also form micropapillary or cribriform structures that fill the gaps separating adjacent papillae. Myoepithelial cells at the periphery of the lesion are also typically absent.2 EPCs express ER and PR, lack HER2 gene amplification, and demonstrate a low to occasionally moderate Ki-67 proliferation index.7 In our case, histopathology confirmed the diagnosis of noninvasive EPC. ER was positive, PR and HER2 were negative, and Ki-67 showed a 22% proliferation rate.
Lymph node metastases have been reported in very rare instances. The incidence of EPC with lymph node involvement is 7%. Two mechanisms were considered to be responsible. First is the cellular displacement of normal or pathologic breast tissue through surgical or needle manipulation and the second cause is embryonic malformation leading to epithelial rests in the lymph node.8 The morphology of metastasis is typically papillary. In our case, 13 axillary lymph nodes were identified, out of which 2 lymph nodes showed micrometastasis, which had papillary features. EPC without invasion should be graded according to its nuclear grade and classified as stage pTis. In the presence of frank invasion, the Nottingham grading, staging, and ER, PR, HER2 status should be determined from the frankly invasive component only. In the presence of nuclear pleo-morphism and increased mitosis, and/or triple negative or HER2 positive phenotype, EPC should be graded, staged, and managed as invasive breast carcinoma.
The differential diagnosis includes other papillary neoplasms like intraductal papilloma, intraductal papilloma with atypical ductal hyperplasia (ADH) or ductal carcinoma in situ (DCIS), papillary DCIS, and solid papillary carcinoma. Intraductal papilloma is histologically characterized by expanded duct composed of arborescent papillae with fibro-vascular core, lined by both epithelial and myoepithelial cells. The peripheries of involved spaces also have myoepithelial cells in contrast to EPC, which lacks myoepithelial cells in the papillae and periphery.
In papilloma with ADH, the papillae show monotonous population of low-grade atypical epithelial cells occupying less than 3 mm. In papilloma with DCIS, intermediate- to high-grade epithelial cells occupy greater than 3 mm. Myoepithelial cells are scant or absent in these areas. Papilloma lacks the capsule, which is present in EPC.
In papillary DCIS, the entire lesion is occupied by a cell population with architectural and cytological features of DCIS. The myoepithelial cells are absent in the papillae and present in the attenuated form at the periphery of the ducts.
Solid papillary carcinomas will have single or multiple nodules with a solid architecture and rudimentary, delicate fibrovascular cores. They lack encircling dense fibrous capsules. Cells may be spindled, producing mucin, and have neuroendocrine features. Similar to EPC, they lack myoepithelial cells within tumor nests and around the periphery.9
The prognosis of EPC without invasion is similar to that of carcinoma in situ. In the absence of associated areas of infiltrating carcinoma, EPC has a very favorable prognosis with adequate local therapy.
Conclusion
EPC of the breast is a rare type of papillary neoplasm. EPC rarely shows lymph node involvement. If lymph node metastasis is present without clear interstitial invasion, the tumor should be considered as self-confined, indolent, invasive carcinoma. Complete surgical excision of the lesion, with extensive sampling of the surrounding breast is essential for treatment and for assessment of local recurrence risk. Histopathology plays a pivotal role in diagnosing this tumor and in identifying the nodal metastasis.
Declaration
The manuscript has been read and approved by all the authors, that the requirements ‘for authorship have been met, and each author believes that the manuscript represents honest work.
Conflict of Interest
None declared.
References
- Garne-lyte A Diagnosis, treatment, and outcomes of encapsulated papillary carcinoma: a single institution experience. Acta Med Litu. 2018;25(02):66-75.
- [Google Scholar]
- Encapsulated papillary carcinoma of breast: clinicopathological features and prognostic parameters. Cureus. 2020;12(10):e11282.
- [Google Scholar]
- Encapsulated papillary carcinoma of the breast: an overview. J Cancer Res Ther. 2013;9(04):564-570.
- [Google Scholar]
- Intracystic (encysted) papillary carcinoma of the breast: a clinical, pathological, and immunohistochemical study. Hum Pathol. 1998;29(10):1097-1104.
- [Google Scholar]
- Encapsulated papillary carcinoma of the breast: a single institution experience. Oncol Lett. 2023;26(04):459.
- [Google Scholar]
- Molecular analysis of encapsulated papillary carcinoma of the breast with and without invasion. Hum Pathol. 2021;111:67-74.
- [Google Scholar]
- Intraductal (intracystic) papillary carcinoma of the breast and its variants: a clinicopathological study of 77 cases. Hum Pathol. 1994;25(08):802-809.
- [Google Scholar]
- Encapsulated papillary carcinoma of the breast: An institutional case series and literature review. Cancer Med. 2023;12(10):11408-11416.
- [Google Scholar]
- Papillary neoplasms of the breast-reviewing the spectrum. Mod Pathol. 2021;34(06):1044-1061.
- [Google Scholar]







