Translate this page into:
Periorbital Ecchymosis With Haemoperitonuem: A Case of Amyloidosis
*Corresponding author: Dr. Adithi Kellarai, Department of General Medicine, K S Hegde Medical Academy, Deralakatte, Mangaluru, India. adithibhandary@nitte.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Kellarai A, Ojha A, Mananje SR, Kishanprasad HL. Periorbital Ecchymosis With Haemoperitoneum: A Case of Amyloidosis. J Health Allied Sci NU. doi: 10.25259/JHASNU_2_2024
Abstract
Amyloidosis is a metabolic storage disorder that results from the deposition of insoluble fibrillar or aberrantly folded proteins in various tissues. It can sometimes present with spontaneous haemorrhage, which in some cases could be life-threatening. Spontaneous intraperitoneal haemorrhage has previously been reported in amyloidosis, but it is extremely rare. We present the case of a patient in her late 60s with diabetes and systemic hypertension, who presented with ecchymosis of both the upper and lower limbs and bilateral periorbital area and a collection in the paracolic gutter. Further investigation confirmed that the retroperitoneal collection was a hematoma. On evaluating the aetiology, the diagnosis of amyloidosis was confirmed via Congo red staining of the abdominal fat pad and bone marrow biopsy, demonstrating characteristic apple-green birefringence under polarised light. The patient was treated conservatively for the hematoma and discharged in a stable condition. This case highlights the rare occurrence of spontaneous intraperitoneal haemorrhage in amyloidosis and emphasises the importance of considering this diagnosis in patients with unexplained hematomas.
Keywords
Amyloidosis
Bone marrow biopsy
Ecchymosis
Haemoperitoneum
Periorbital
INTRODUCTION
Amyloidosis is a rare metabolic storage disorder that results from the deposition of insoluble aberrantly folded proteins in various tissues. The presentation depends upon the type of amyloidosis, its onset, and the extent of organ involvement. Although the disease is rare, it has a high morbidity and mortality. In certain cases, mortality may be seen within 6 months of diagnosis.[1,2] Disease symptomatology can vary from mild symptoms like fatigue, weight loss, and discolouration of the limbs to severe complications like left ventricular failure, nephrotic range proteinuria with renal parenchymal damage, liver failure, and vasculitis of various organs.[3] Patients may sometimes present with spontaneous haemorrhage. Haemorrhage can range from mild symptoms, such as easy bruising, purpura, and mucosal bleeding, to more severe spontaneous haemorrhage in the gastrointestinal or renal system. This manifestation can be attributed to amyloid deposition within vascular walls, leading to vessel fragility. Other factors contributing to haemorrhage include thrombocytopathy, coagulopathy, and factor deficiency.[4] Understanding bleeding manifestations is imperative to assess and comprehend disease complexity. However, the full spectrum of bleeding complications and their pathophysiological mechanisms in amyloidosis remain areas of ongoing research.
Spontaneous intraperitoneal haemorrhage is a rare and life-threatening manifestation of amyloidosis. It requires a high index of suspicion for early diagnosis, especially in patients with unexplained abdominal symptoms. Due to its varied symptoms and rarity, diagnosing amyloidosis can be challenging, and it is often under-recognised. Clinical data regarding the exact pathophysiology and presentation of spontaneous intraperitoneal haemorrhage is limited in India. Here, we discuss the case of a patient who presented with a two-week history of abdominal distention, constipation, and abdominal pain, who was found to have a spontaneous intraperitoneal bleed upon evaluation. Further investigation led to a diagnosis of amyloidosis. Our case underscores the importance of considering a diagnosis of amyloidosis in patients who present with unexplained haemorrhage or systemic symptoms involving cardiac, renal, or skin and highlights the need for a comprehensive diagnostic approach.[5]
CASE REPORT
We present a female patient in her late 60s who presented to the emergency room with complaints of abdominal distension of 15 days duration. It was insidious in onset, gradually progressive, and associated with constipation. She also developed abdominal pain lasting three days, which was a dull aching type, in the left iliac fossa, radiating to the back, pain lasting throughout the day with no aggravating or relieving factors. She had no history of vomiting, fever, leg swelling, facial oedema, decreased urine output, or cardiorespiratory symptoms. She had no history of trauma. She visited a local hospital with these complaints and was diagnosed with anaemia and received 2 units of packed cell transfusion. During her stay there, she developed breathlessness and was shifted to an ICU, where she required non-invasive ventilation. She was diagnosed to have acute coronary syndrome with pulmonary oedema and treated with antiplatelets and diuretics. She was a known case of Type 2 Diabetes mellitus for 20 years on medication, systemic hypertension for five years on medications, and anaemia on hematinic for 2-3 years and was recently diagnosed with chronic kidney disease.
On presentation, the patient was stable with a pulse of 90 beats/min and blood pressure of 110/60 mmHg. A general physical examination of the patient revealed pallor and bilateral pitting pedal oedema. Jugular venous pressure was normal. A head-to-toe examination revealed bluish discoloration around the bilateral periorbital area, upper limb, and left lower limb ecchymosis. Abdominal examination showed a uniformly distended abdomen with all quadrants moving equally with respiration, an inverted umbilicus without dilated veins/scars/sinuses. On palpation, there was tenderness present over the left iliac fossa with no guarding and rigidity. Percussion revealed shifting dullness suggestive of ascites, no fluid thrill, and on auscultation, bowel sounds were heard. Respiratory system examination revealed bilateral decreased breath sounds in the axillary, infra-axillary, and infra-scapular areas suggestive of pleural effusion. Cardiovascular and central nervous system examinations were normal. The patient was admitted to the ICU for management of severe anaemia.
Blood investigations demonstrated reduced haemoglobin (6.6 g/dL), leucocytosis (28650 cells/mm*3), and normal platelets. All bleeding parameters were normal. Peripheral smear revealed dimorphic anaemia. Iron indices revealed a low total iron binding capacity (252 µg/dL), increased serum iron (175 µg/dL), and increased ferritin (1860 ng/mL). Renal function test was normal except for hyponatremia, and liver function test revealed hypoalbuminemia (2.3 g/dL) and mildly elevated liver enzymes. A urine analysis revealed the presence of leukocytes and albumin. Further, a culture sensitivity of the urine revealed E. coli, for which the patient was initiated on antibiotics. Due to the presence of albumin in urine, a 24-hour urine protein: creatinine (11.34) test was conducted. Ascitic fluid analysis showed ascitic fluid protein (3.4 g/dL), ascitic fluid albumin (1.4 g/dL), and serum ascites albumin gradient (SAAG 0.9), with no features of peritonitis. An ultrasound (USG) abdomen showed ascites and paracolic gutter collection. A contrast-enhanced computed tomography (CECT) of the abdomen revealed a collection in the paracolic gutters and left psoas muscle [Figure 1]. In view of these findings, an initial suspicion of psoas abscess was made. USG-guided percutaneous drainage from the paracolic collection revealed blood with a negative culture report ruling out an abscess. Therefore, a revised diagnosis of hematoma was considered. In view of spontaneous ecchymosis around the eyes and skin of limbs, a suspicion of amyloidosis was made, and an abdominal fat pad biopsy was performed. This test confirmed the diagnosis [Figure 2]. In view of severe anaemia, a bone marrow aspiration and biopsy were performed. Bone marrow aspiration was suggestive of hyperplastic marrow with erythroid and granulocytic hyperplasia and plasmacytosis (9%). Bone marrow biopsy was faintly positive for the Congo red stain, and features were suspicious of marrow involvement by amyloidosis [Figure 3]. Comprehensive myeloma protein panel (CMPP) for multiple myeloma workup showed an absence of the M BAND and no evidence of monoclonal gammopathy on protein electrophoresis. ĸ and λ bands were not detected in serum immunofixation electrophoresis, and the ĸ/λ ratio was 0.78 (normal). Total IgG was 17.30 g/L, and β2 macroglobulin was 6663 ng/mL, which were high. In view of the nephrotic range proteinuria, a kidney biopsy was performed, revealing diabetic nephropathy. An upper gastrointestinal endoscopy was done as a workup for anaemia, which revealed gastritis. A 2D echo revealed a speckled appearance of the interventricular septum, thickened interatrial septum (9 mm) with mild mitral regurgitation, and mild pulmonary arterial hypertension with preserved ejection fraction (EF - 60%). Patient was also suggested a cardiac MRI to rule out cardiac involvement of amyloidosis, which the patient refused. NT pro-BNP (N-terminal pro B-type natriuretic peptide) was high (10468 pg/mo).
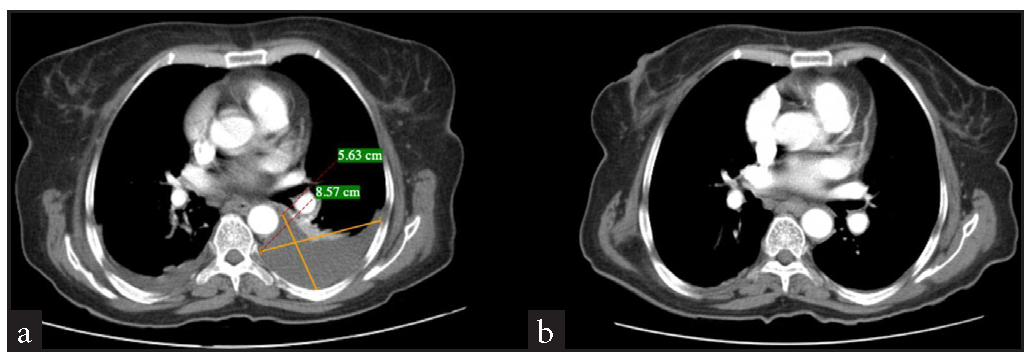
- Contrast CT abdomen and pelvis. (a) Contrast CT abdomen reveals a psoas collection (8.57cm *5.63 cm) communicating with the left paracolic gutter. (b) Contrast CT abdomen after the aspiration of the collection.
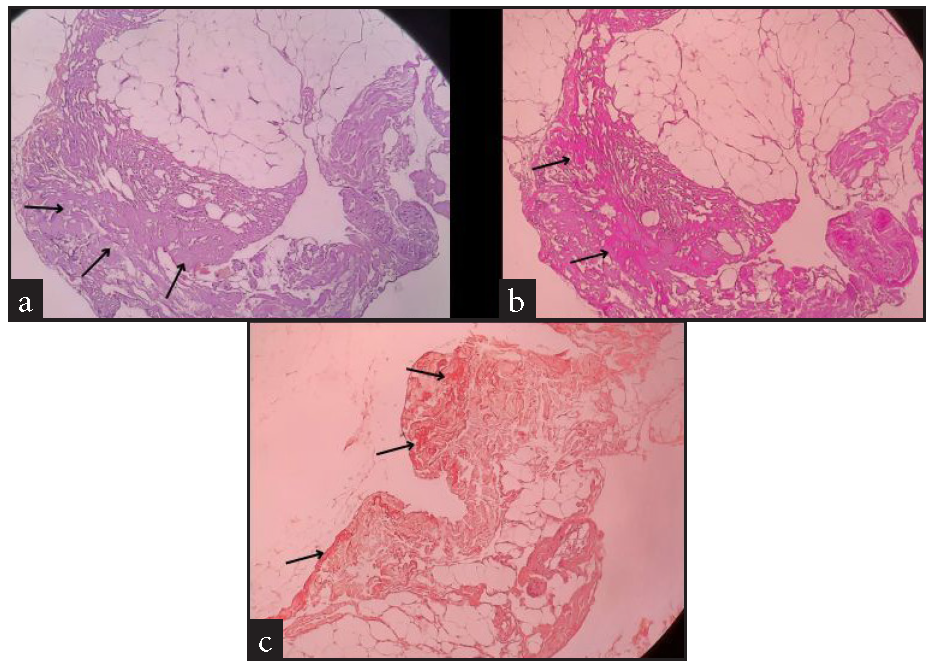
- Histopathology of abdominal fat pad biopsy under different stains suggestive of amyloidosis (a) Haematoxylin and eosin shows metachromatic stain (purple) around the blood vessels & also in homogeneous acellular areas showing amyloid material (black arrow). (b) Periodic acid Schiff stain showing amyloid material (black arrow). (c) Congo red stain showing amyloid material (black arrow).
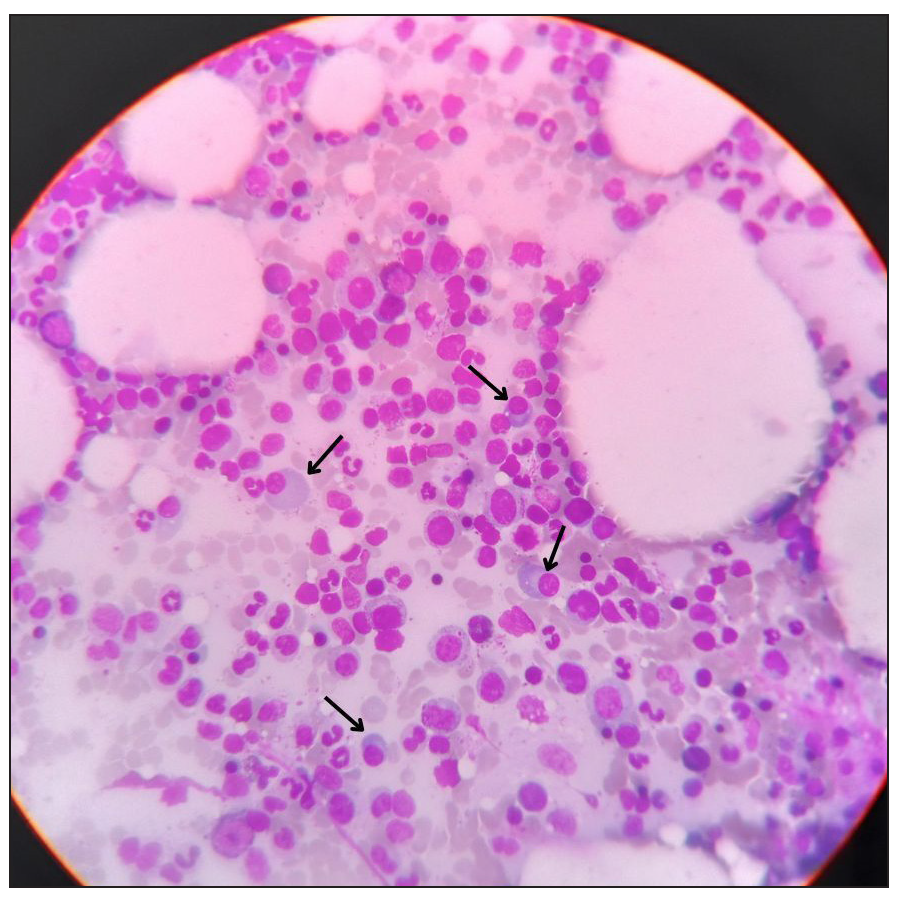
- Microphotograph of bone marrow aspiration under Giemsa’s stain with both erythroid and myeloid hyperplasia displaying plasmacytosis (black arrow).
Initially, the patient was treated for sepsis with antibiotics, heart failure was managed with diuretics, and packed cell transfusion was given to correct anaemia. Following three days of ICU care, the patient felt symptomatically better and was shifted to the ward. The patient was conservatively treated for the hematoma, which resolved on a review USG scan. The patient was advised tissue typing for diagnosis of a subtype of amyloidosis, but they were unwilling for further workup or treatment, and she was discharged with antidiabetic and anti-hypertensive medications.
DISCUSSION
Amyloidosis is a complex condition that arises from the abnormal deposition of amyloid protein in various tissues and organs, leading to significant morbidity and mortality. Amyloidosis can affect individuals across all age groups, but certain forms are more prevalent in specific age ranges. The age distribution of amyloidosis exhibits significant variation depending on its type and underlying etiological factors. Among systemic forms, such as Light-Chain (AL) and Transthyretin (ATTR) amyloidosis, the average age at diagnosis tends to cluster in older populations, often between ages of 50 and 70, whereas serum amyloid A amyloidosis (AA) is more commonly observed in younger patients, particularly those with chronic inflammatory conditions.[6] Research indicates variability in male and female prevalence across different types of amyloidosis, particularly AL, where men exhibit a higher incidence. Specifically, some studies report a male-to-female ratio of approximately 1.5:1.[7]
Clinical presentation is highly variable depending on the type of amyloidosis. Patients may present with vague symptoms such as fatigue and weight loss. AA type is seen as being associated with chronic infections and inflammation, hence showing greater prevalence in developing countries.[8] Primary amyloidosis (AL type) can present with left ventricular hypertrophy and heart failure; nephrotic syndrome is also observed in this, the kidney being the 2nd most common organ affected after the heart. An enlarged tongue, along with skin manifestations like periorbital purpura, may present as a classical finding.[9] Spontaneous haemorrhage in amyloidosis can range from 15-40%. The presentation of gastrointestinal disease depends on the type of amyloidosis. It is more common in AA amyloidosis compared to AL Amyloidosis. However, gastrointestinal bleeding has been described in both sets of patients.[4]
Spontaneous haemoperitoneum is a clinically significant condition marked by the unexpected accumulation of blood in the peritoneal cavity without any obvious external injury or surgical intervention. Understanding its aetiology has potential implications for patient outcomes. Several aetiologies have been proposed to explain the origins of spontaneous haemoperitoneum, including ruptured vascular malformations (aneurysm and pseudoaneurysm), Ehlers-Danlos syndrome, increased small vessel fragility, ectopic pregnancy, rupture of an ovarian cyst, hepatic tumours or liver disease including HELLP syndrome, and some less common causes includes pancreatitis.[10] Spontaneous haemorrhage is a rare but recognised complication of amyloidosis; it can occur due to a number of factors. Primarily, haemorrhage is attributed to vascular infiltration by amyloid fibrils, leading to endothelial dysfunction and increased fragility. In most of these cases, there is no coagulation deficiency. In our patient, the prothrombin time and activated partial thromboplastin time were normal, suggesting the possibility of haemorrhage secondary to amyloid deposit. However, we did not test individual clotting factors in our patient. Platelet dysfunction, deficiency of coagulation factor, and increased fibrinolysis may be additional predisposing factors for spontaneous bleeding.[4,11,12]
Clinical presentation of spontaneous intraperitoneal haemorrhage can be diverse and often nonspecific, complicating the diagnostic process. Similar to what was seen in our case, the common symptoms include acute abdominal pain, distension, and signs of hypovolemic shock, such as hypotension and tachycardia. The clinical presentation can be subtle, and the diagnosis may be delayed, especially in the absence of a known history of amyloidosis.[13] Amyloidosis can also manifest as gastrointestinal disturbances, including diarrhoea, weight loss, and abdominal pain due to amyloid deposits in the intestinal tract.[14]
When considering a diagnosis of amyloidosis as an aetiology for haemoperitoneum high index of suspicion is warranted. It is crucial to rule out other potential aetiologies of hematoma before attributing haemorrhage to amyloidosis. Diagnosing spontaneous intraperitoneal bleeding in the context of amyloidosis involves a combination of clinical evaluation, imaging studies, and laboratory tests. Confirmatory diagnosis of amyloidosis typically requires a tissue biopsy, with subsequent histological examination demonstrating amyloid deposits. Immunohistochemical staining can help identify the type of amyloid protein involved.[15] In our case, we investigated the patient for other causes of hematoma, including coagulopathies, perforation, and trauma, before concluding the association with amyloidosis. Our case highlights the necessity for thorough investigations using imaging techniques such as USG and computed tomography (CT), which can reveal the aetiology, size, and extent of hematomas, while laboratory evaluations might identify underlying anaemia, thrombocytopenia, coagulopathy, or inflammatory markers.
The prognosis for patients with spontaneous intraperitoneal bleeding due to amyloidosis depends on the severity of the bleeding, the extent of organ involvement, and the promptness of diagnosis and intervention. Management may include supportive care, treatment of the coagulation deficiency, and, in certain cases, surgical intervention. Literature suggests that treatment of patients with chemotherapy and other targeted therapies may decrease bleeding tendencies in the future.[4,16] Hence, early diagnosis and treatment may improve the outcome of the patient.
Our case highlights the importance of diligent screening of rare amyloidosis-related complications, such as spontaneous intraperitoneal haemorrhage in patients presenting with unexplained abdominal pain and distension. Additionally, it is essential to consider other potential causes of spontaneous intraperitoneal haemorrhage and develop a personalised therapeutic plan to manage these patients effectively.
CONCLUSION
Amyloidosis has multisystemic manifestations, necessitating comprehensive invasive and non-invasive investigations for accurate diagnosis. Spontaneous intraperitoneal haemorrhage is a rare and life-threatening complication, and delays in recognition can lead to poor outcomes. Enhanced diagnostic tools, such as advanced imaging techniques (Dual energy computed tomography, magnetic resonance imaging) and biomarker analysis (NT-proBNP, serum amyloid A), should be employed for early detection and timely intervention to improve patient prognosis.
Acknowledgement
The completion of this case report would not have been possible without the support of the dean of my institution, Prof Dr P.S. Prakash. The encouragement and insightful feedback were instrumental in accomplishing this task.
I thank the staff of pathology, nephrology, and interventional radiology for their cooperation and kind support throughout this period.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Natural history and outcome in systemic AA amyloidosis. N Engl J Med. 2007;356:2361-71.
- [CrossRef] [PubMed] [Google Scholar]
- Global epidemiology of amyloid light-chain amyloidosis. Orphanet J Rare Dis. 2022;17:278.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Light chain amyloidosis: Epidemiology, staging, and prognostication. Methodist DeBakey Cardiovascular Journal. 2022;18:27-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Amyloidosis and bleeding: Pathophysiology, diagnosis, and therapy. Am J Kidney Dis. 2006;47:947-55.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic amyloidosis journey from diagnosis to outcomes: a twelve-year real-world experience of a single center in a middle-income country. Orphanet J Rare Dis. 2022;17:425.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Diagnosis and treatment of hereditary transthyretin amyloidosis (hATTR) polyneuropathy: Current perspectives on improving patient care. Ther Clin Risk Manag. 2020;16:109-23.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Dysregulation of miRNAs in AL amyloidosis. Amyloid. 2011;18:128-35.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Systemic AA amyloidosis: epidemiology, diagnosis, and management. Clinical Epidemiology. 2014;6:369-77.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- AL Amyloidosis: Unfolding a complex disease. Journal of the Advanced Practitioner in Oncology. 2019;10:813-825.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Spontaneous abdominal hemorrhage: Causes, CT findings, and clinical implications. AJR Am J Roentgenol. 2009;193:1077-87.
- [CrossRef] [PubMed] [Google Scholar]
- Thromboembolism and bleeding in systemic amyloidosis: a review. ESC Heart Failure. ;9:11-20.
- [CrossRef] [Google Scholar]
- Amyloidosis presenting with platelet dysfunction and recurrent retroperitoneal hemorrhage (RH) Blood. 2007;110:3944.
- [CrossRef] [Google Scholar]
- Idiopathic spontaneous intraperitoneal haemorrhage: A near fatal presentation of acute abdomen requiring prompt diagnosis. Int J Surg Case Rep. 2023;110:108650.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Gastrointestinal: Diarrhea and bowel thickening in elderly patients: A diagnosis to keep in mind. J Gastroenterol Hepatol. 2021;37:418.
- [CrossRef] [PubMed] [Google Scholar]
- Case report: a case of AL amyloidosis with spontaneous giant retroperitoneal hematoma. Int J Emerg Med. 2024;17:200.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Successful treatment of systemic amyloidosis with hepatic involvement and factor X deficiency by high dose melphalan chemotherapy and autologous stem cell reinfusion. Eur J Haematol. 2004;72:181-185.
- [CrossRef] [PubMed] [Google Scholar]







